GLOBAL PATIENT SUPPORT PROGRAM
Life saving drugs are either beyond the reach of general people or not available in many countries. Because of the patient exemptions for Bangladeshi companies,Beacon Pharmaceuticals can manufacture the patented drugs. As a support to global patient.
REGISTERED & LICENSED BY REGULATORY AUTHORITY OF BANGLADESH
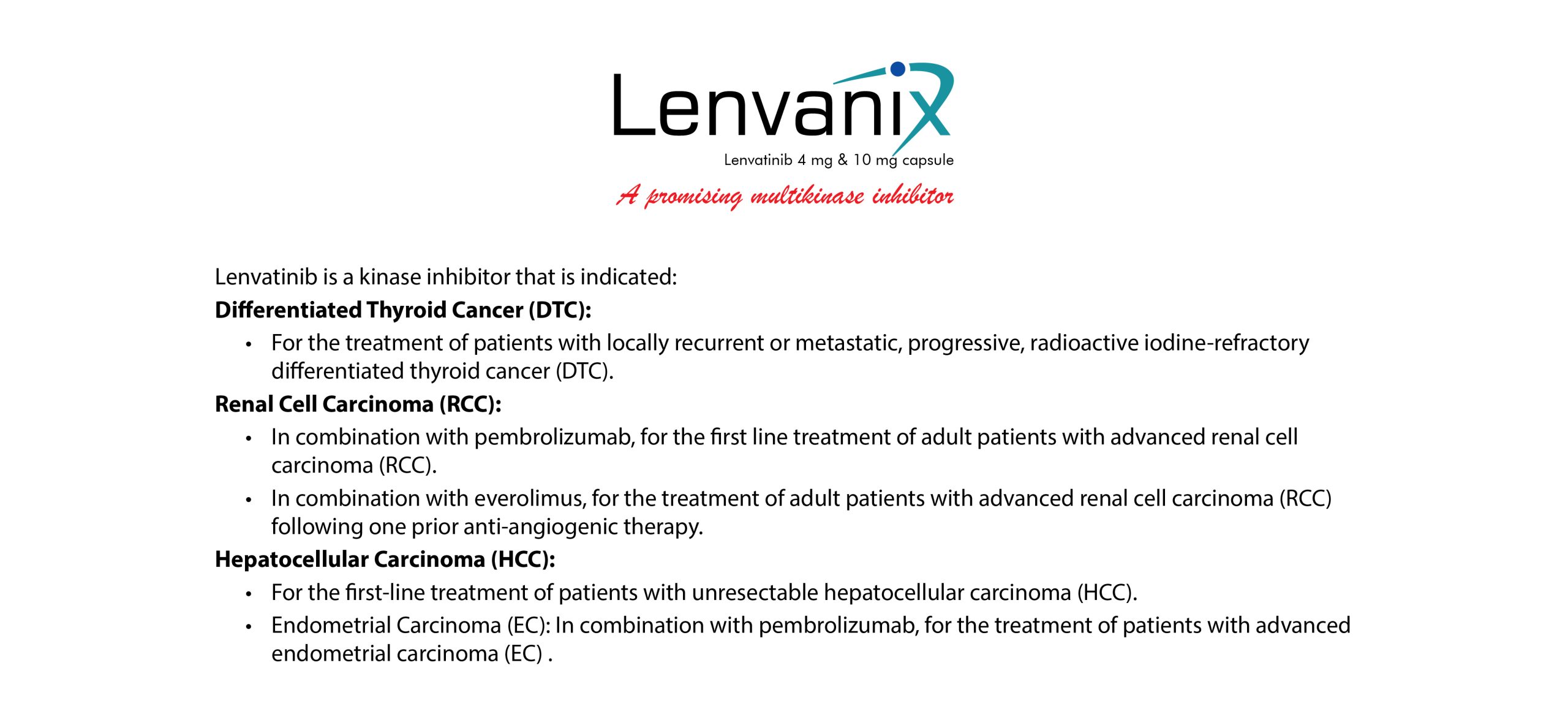
Lenvanix is a registered product by the Directorate General of Drug Administration & Licensing Authority (Drugs) of Bangladesh.
Lanvanix 4
Drug Marketing Authorization: 341-333-010
Inclusion Date: 24-04-2018 Valid up to: 25-04-2028
Lanvanix 10
Drug Marketing Authorization: 341-334-010
Inclusion Date: 24-04-2018 Valid up to: 23-04-2028